Bipolar membranes are a crucial component in various energy technologies, such as electrolyzers and hydrogen fuel cells. These membranes consist of two layers, the cation-exchange and anion-exchange layers, which are essential for ion conduction. Despite the widespread use of bipolar membranes in the industry, there is still a lack of comprehensive understanding regarding their working principles and ion solvation kinetics.
A recent study conducted by researchers at the Fritz-Haber Institute of the Max Planck Society aimed to investigate the water dissociation and ion solvation kinetics at the interface between the two layers in bipolar membranes. The study, published in Nature Energy, provided valuable insights that could potentially revolutionize the future design and fabrication of these membranes and electrocatalysts for fuel cells.
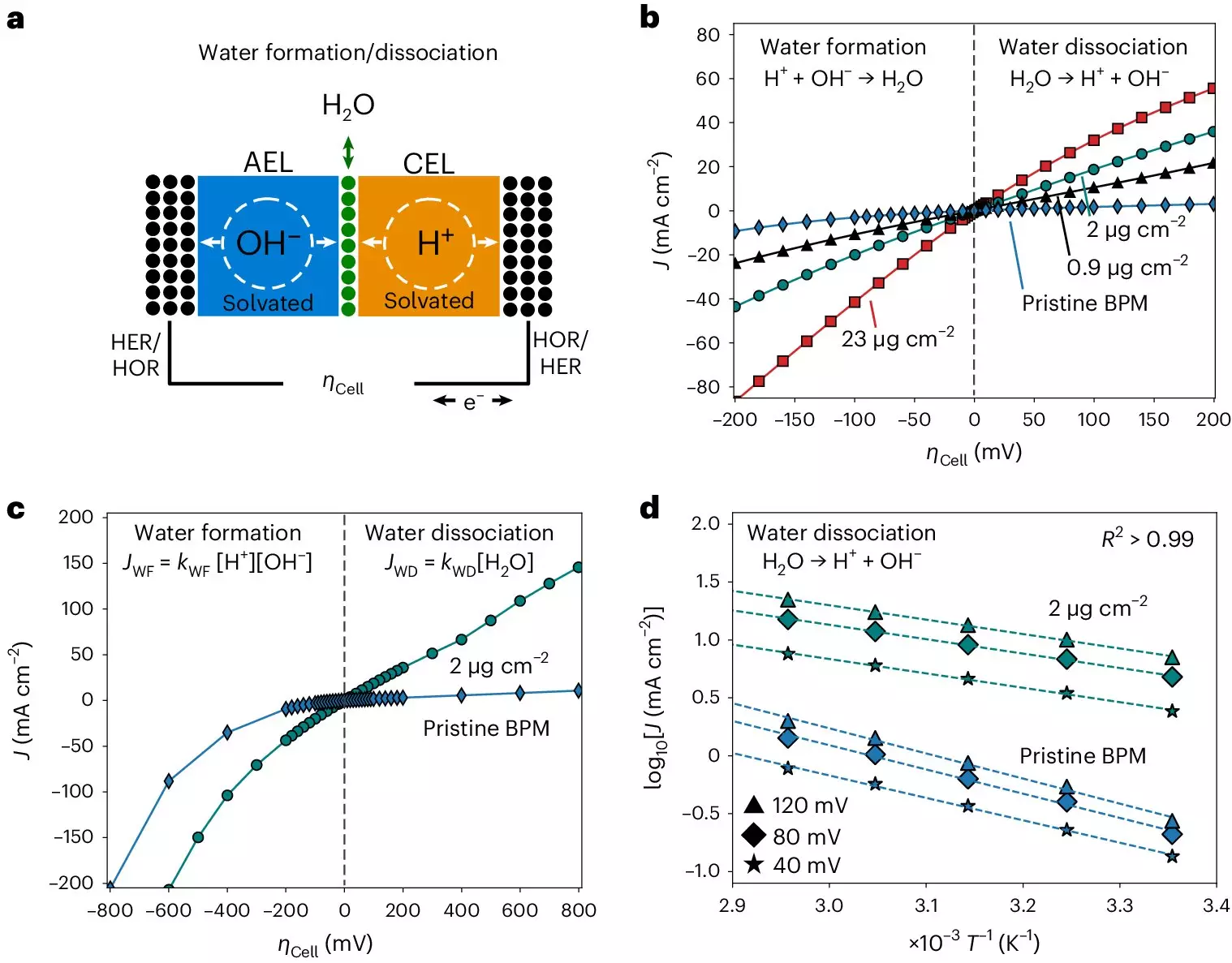
The research team, led by Sebastian Oener and Carlos Gomez Rodellar, faced various challenges in setting up an experimental system to study the kinetics of bipolar membranes accurately. The researchers discovered bias-dependent relationships between the activation entropy and enthalpy inside the bipolar junction, indicating a connection to interfacial capacitance. Moreover, they observed that solvation kinetics in bipolar membranes are influenced by entropic changes in the interfacial electrolyte rather than the catalysts used.
The insights gained from the study could have significant implications for the development of more efficient bipolar membranes for applications such as electrodialysis, CO2 electrolyzers, and H2 fuel cells. Oener emphasized the vast potential of bipolar membranes in various energy-related fields and highlighted the importance of understanding the physics behind water dissociation and ion solvation at electrocatalyst interfaces.
While the study shed light on the fundamental principles of bipolar membranes, there are still unresolved questions that the researchers aim to address. The formation of water during the operation of bipolar membranes in the forward direction remains a critical aspect that requires further investigation. Additionally, the team is exploring collaborations with other institutions to expand the applications of bipolar membranes in different types of fuel cells and electrodialysis systems.
The study conducted at the Fritz-Haber Institute provides essential insights into the working mechanisms of bipolar membranes and their potential applications in energy technologies. By unraveling the ion solvation kinetics and entropic changes at liquid-solid interfaces, the researchers hope to pave the way for the development of more efficient electrocatalysts and green energy solutions.

Leave a Reply