Lithium-metal batteries have long been hailed as the next frontier in battery technology, offering significantly higher energy densities than the current lithium-ion batteries that dominate the market. However, despite their potential, lithium-metal cells have been plagued by a number of limitations, particularly in terms of their short lifespan. Researchers at the University of Science and Technology of China and other institutes have recently made significant strides in addressing this issue by introducing a new electrolyte design that could pave the way for highly performing lithium-metal pouch cells with longer lifespans.
One of the main challenges faced by lithium-metal batteries is their limited cycle life, with existing cells typically lasting only around 50 cycles compared to the 1,000 cycles that commercial lithium-ion batteries can achieve. The primary reasons behind this lower lifespan are the growth of lithium dendrites, the high reactivity of lithium-metal, and high-voltage transition metal cathodes, all of which contribute to the constant degradation of the electrolyte. Despite tireless efforts by researchers worldwide, the performance of lithium-metal batteries has remained unsatisfactory, falling short of the desired energy density and cycle life.
Approximately five years ago, Prof. Shuhong Jiao and her colleagues embarked on a journey to develop an electrolyte that could stabilize the interfaces between the electrolyte and electrodes, thus suppressing degradation in lithium-metal battery cells. Their groundbreaking electrolyte design builds on a deep understanding of the microscopic physicochemical processes that govern the behavior of lithium-metal batteries. By focusing on the critical role of the electrolyte in dictating battery performance, the research team sought to create a solution using affordable components while drawing inspiration from the works of other researchers in the field.
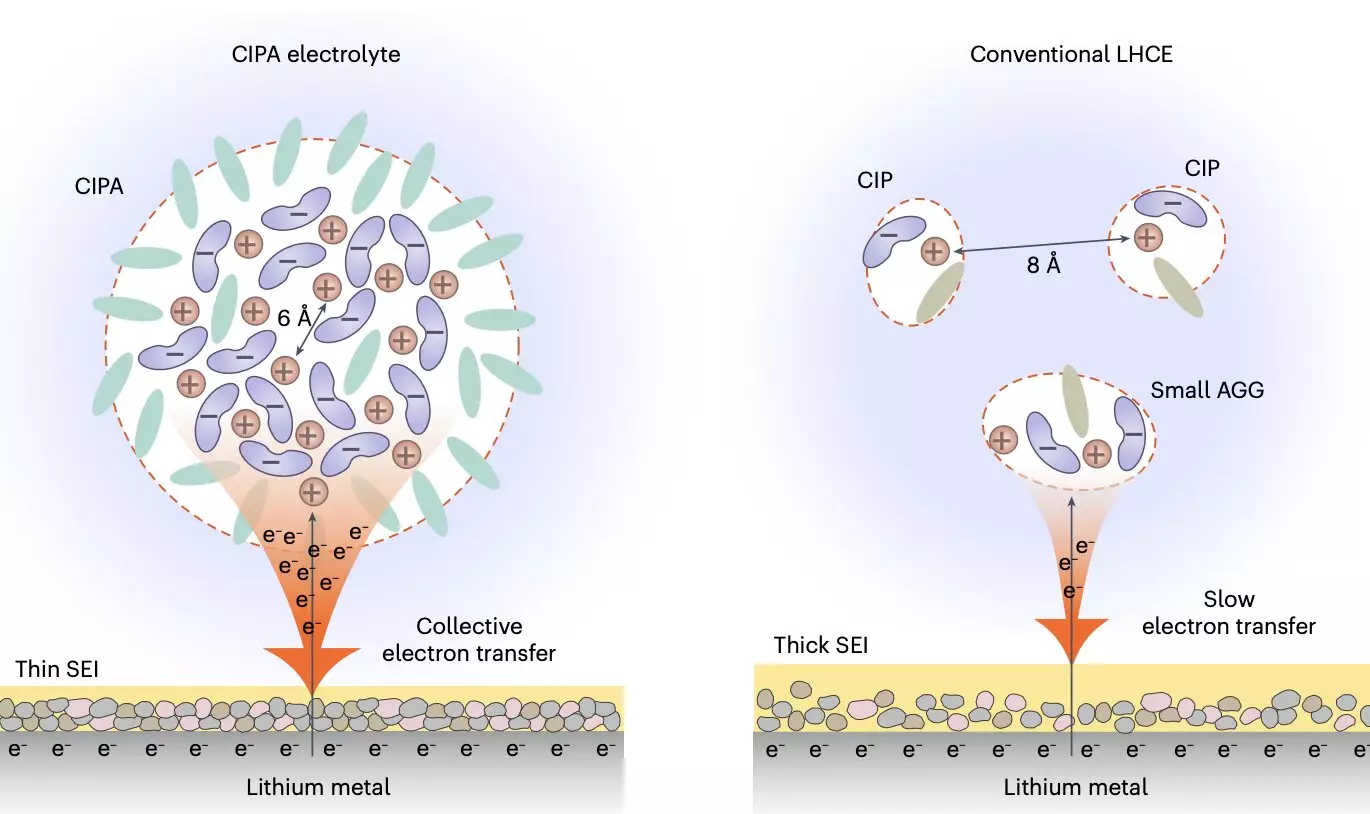
In their recent study, Prof. Jiao and her team collaborated with experts in theoretical calculations and electrolyte characterization to design a new class of electrolytes that can significantly extend the lifespan of lithium-metal batteries. These unique electrolytes are characterized by their mesoscopic solvation structure, with a particular focus on the interaction between ion pairs that underlie the formation of the electrolyte’s aggregate structure. By introducing large compact aggregates known as ‘compact ion-pair aggregates (CIPA),’ the researchers were able to achieve a stark contrast to existing electrolyte designs, opening up new possibilities for electrolyte optimization.
The new electrolyte design introduced by Prof. Jiao’s team exhibits a collective reduction on the lithium-metal anode, leading to the rapid formation of a thin and stable solid electrolyte interface (SEI) that suppresses electrolyte decomposition. This unique collective electron transfer behavior results in a homogeneous and compact lithium deposition, significantly reducing the specific areas of the lithium-metal anode and further enhancing stability. Additionally, the electrolyte demonstrates good oxidative stability and effectively suppresses the dissolution of transition metal elements from the cathode, resulting in improved cycling stability and performance.
The development of this advanced electrolyte marks a significant milestone in the quest for high-performance lithium-metal batteries. As researchers continue to explore the potential of this new electrolyte design, there is hope for further advancements in energy density and cycle life. The creation of a 500 Wh/kg lithium-metal pouch cell that retains 91% of its energy after 130 cycles is a testament to the promising future of this technology. With plans to extend the cycle life of these cells to over 1,000 cycles and explore even higher energy densities, the future of lithium-metal batteries looks brighter than ever.

Leave a Reply