As global temperatures rise and the effects of climate change become increasingly evident, the need for sustainable energy solutions has never been more urgent. One promising avenue lies in unearthing innovative methods of recycling carbon dioxide (CO2) to create sustainable fuels. Emerging research from the University of Michigan highlights an artificial photosynthesis system that has made significant strides in this field, potentially transforming the way we produce essential hydrocarbons and addressing CO2 emissions concurrently.
This breakthrough technology is particularly noteworthy for its ability to efficiently bind carbon atoms, achieving a performance standard that significantly outpaces existing artificial photosynthesis systems. The system specializes in producing ethylene—a key hydrocarbon utilized predominantly in the plastics industry. By capturing CO2 that would have otherwise contributed to atmospheric pollution, this novel approach proposes a circular economy of carbon use that could reshape environmentally damaging industries.
Zetian Mi, a prominent professor of electrical and computer engineering and the lead researcher on the project, reveals that the system’s performance metrics are astonishingly superior to conventional methods of solar-driven CO2 reduction. It boasts an efficiency and stability rating that is five to six times higher than typical standards, positioning it as a leader in the domain of artificial photosynthesis. Ethylene, being the most produced organic compound globally, is predominantly manufactured through carbon-intensive fossil fuel processes, emitting CO2 and other pollutants in the process.
The importance of transitioning from fossil-based methods to sustainable alternatives cannot be overstated. The strategic application of this new system aims not only to produce ethylene but also to pave the way for longer carbon chains that can synthesize liquid fuels, offering a viable alternative to conventional fuel sources. Producing fuels in a sustainable manner would not only address environmental concerns but also facilitate the integration of renewable practices into existing energy infrastructures.
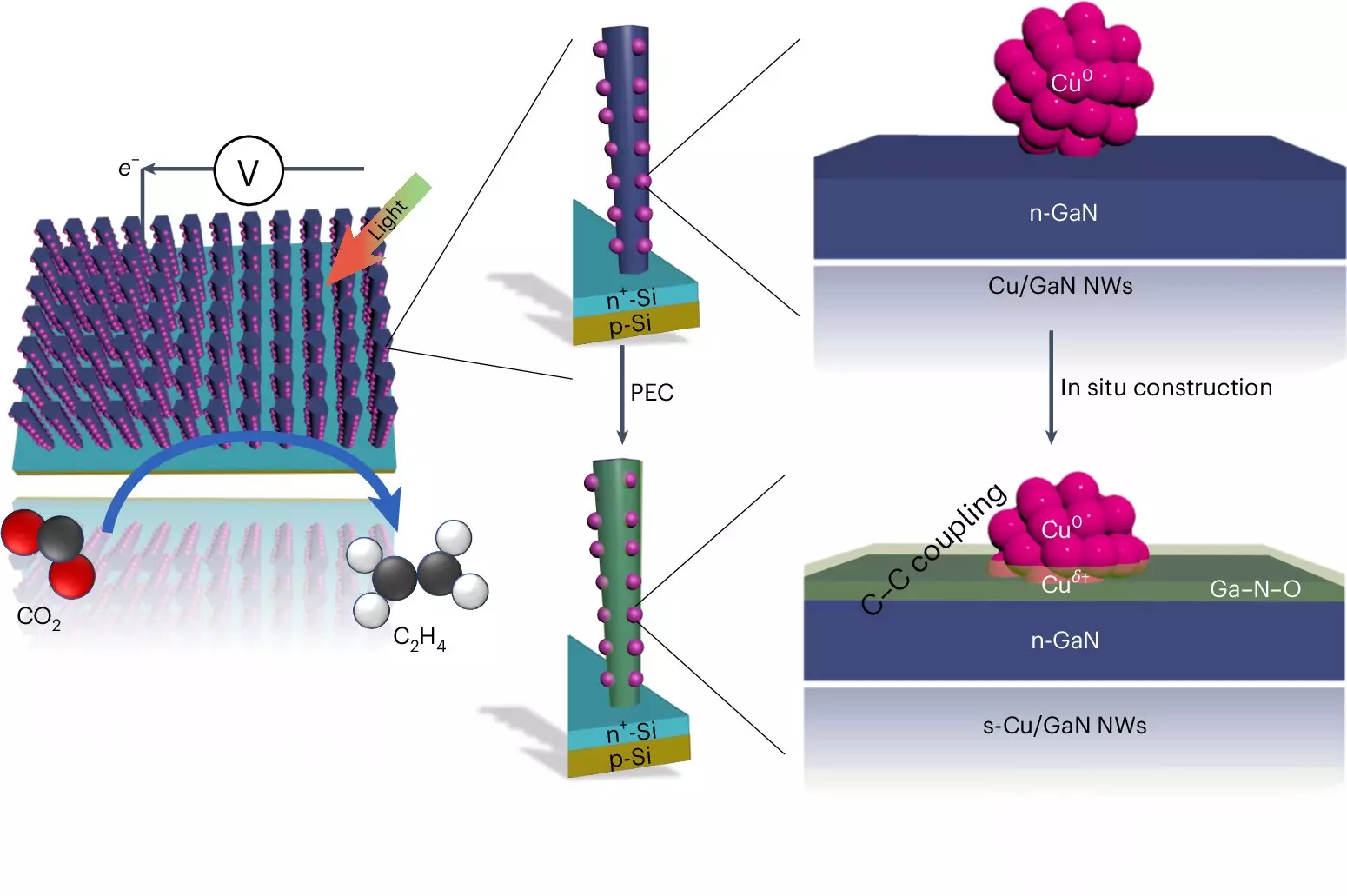
At the heart of this artificial photosynthesis system lies a sophisticated mechanism that engages two types of semiconductors: gallium nitride nanowires and a silicon substrate. The incredible finesse of these nanowires, which are only a few hundred atoms wide, enables them to absorb sunlight effectively. When illuminated, the system initiates a series of reactions where water and CO2 interact to synthesize ethylene. The key to this process is the copper clusters situated along the nanowires, each composed of approximately thirty atoms.
This intricate sequence begins with sunlight energizing the nanowires, resulting in the splitting of water molecules. This reaction yields hydrogen—an essential component in the transformation of carbon dioxide into ethylene—while producing oxygen that is subsequently converted into gallium nitride oxide. It is here that the copper clusters play a pivotal role, efficiently capturing hydrogen and facilitating the bonding of two carbon monoxide molecules, ultimately leading to ethylene production.
What sets this new method apart is not just its ability to efficiently convert CO2 into ethylene, but its remarkable longevity and operational stability. The Michigan team reported that their device could continuously operate for an impressive 116 hours without any decline in performance—an achievement that eclipses other catalytic processes that typically degrade within a matter of hours. This durability can be attributed to the beneficial interactions between gallium nitride and the water-splitting mechanism, allowing for a self-healing process that enhances overall efficiency.
The efficacy of the Michigan system reflects a considerable leap forward, as it produces ethylene at more than four times the rate of its nearest competitors. While alternative methods have shown promise, they often fall short in either duration or efficiency, requiring carbon-based fluids or falling victim to rapid degradation. The implications of such a breakthrough could redefine standards in artificial photosynthesis technology, establishing a blueprint for future innovation.
Keen to expand on this foundation, the researchers at the University of Michigan plan to target the synthesis of more complex hydrocarbon compounds, including propanol—a three-carbon alcohol. The broader objective of producing liquid fuels will also enable various transportation technologies to transition to more sustainable models.
As we grapple with the consequences of climate change and fossil fuel dependence, the quest for cleaner, renewable energy solutions takes precedence. The University of Michigan’s advancements in artificial photosynthesis epitomize the spirit of innovation necessary to tackle these pressing challenges, indicating a transformative potential that may one day lead to significant reductions in global CO2 emissions. Such strides in technology not only promise a greener future but also cultivate hope for a more sustainable and resilient energy landscape.

Leave a Reply