The study of physical systems often reveals surprising parallels with biological phenomena. One compelling example is how classical mixture theory, typically reserved for physics, can be applied to understand the intricate processes within living cells. At São Paulo State University (UNESP) in Brazil, researchers are pioneering work that models protein compartmentalization as a form of cellular Griffiths-like phase. This approach offers deep insights into cellular dynamics and could even shed light on life’s origins.
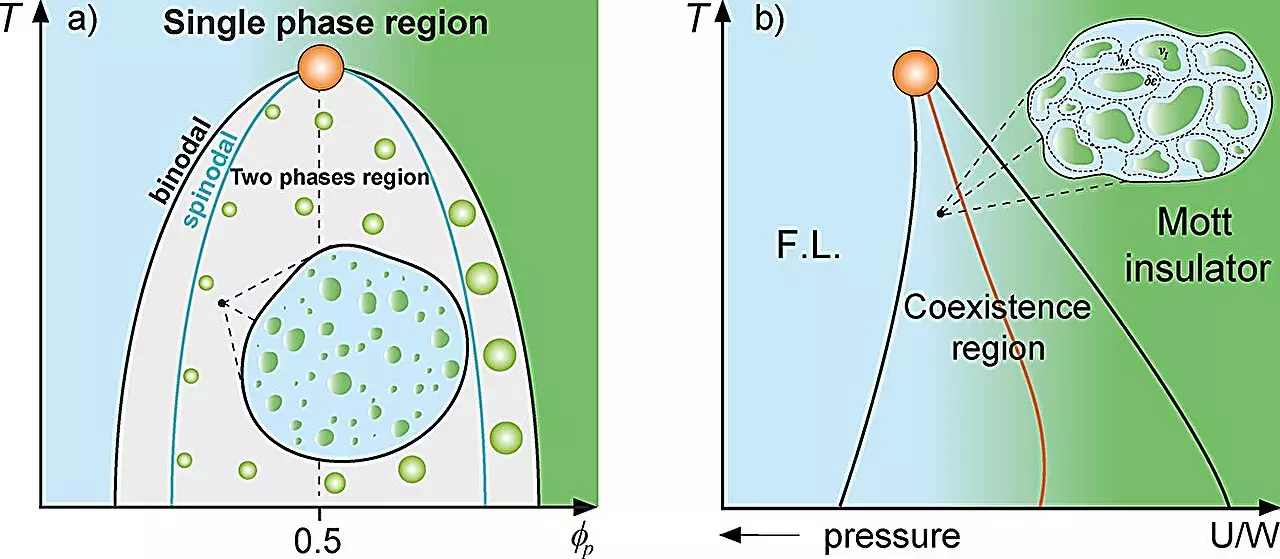
In classical physics, mixture theory explores how different phases can coexist in a single system, as seen in supercooled water or the Mott metal-insulator transition. The concept of “rare regions” within these systems—areas that exhibit different properties compared to their surroundings—serves as a useful analogy for understanding how proteins behave within cells. The leading figures in this research, Mariano de Souza and Lucas Squillante, present a framework that draws from condensed matter physics to analyze biological interactions at the cellular level.
The Griffiths phase is a concept that arises in magnetic systems, highlighting the emergence of magnetized and non-magnetized zones within a matrix. Such regions, occurring randomly, can lead to significant changes in a system’s dynamics. This concept has been broadened by Souza’s team to describe protein dynamics as influenced by their concentration and phase separation within cells.
When proteins exceed a certain threshold concentration, they can undergo liquid-liquid phase separation, resulting in the formation of distinct compartments or droplets. The researchers applied thermodynamic models, including the Flory-Huggins and Avramov-Casalini models, to illustrate how cellular dynamics change in proximity to these phase separations. Notably, they demonstrate that a Griffiths-like cellular phase reduces overall protein mobility, a finding that could have profound implications for understanding gene expression and cellular function.
An intriguing aspect of this research is its connection to the origins of life, guided by the classical theories posed by Aleksandr Oparin in the 1930s. Oparin suggested that life began with simple organic molecules clustering together, a notion that aligns with the coacervates discussed in the current study. By proposing that only those droplets with slower dynamics were able to survive and evolve, the researchers intertwine the principles of physical chemistry with biological evolution.
Another significant component of their findings is chirality—an essential property in biological molecules. The predominance of one chiral form over another plays a critical role in biochemical processes. Souza’s work suggests that dynamics and fluctuations within these protein compartments can influence chirality, which is a foundational element in the evolution of biological systems.
As the research unfolds, the implications for understanding and treating diseases become increasingly apparent. From tumorigenesis to neurodegenerative disorders, the compartmentalization of proteins via phase separation is a hot topic in current scientific discourse. For instance, certain diseases are linked with the way proteins aggregate in specific cellular environments, ultimately affecting their functionality.
Minicucci, a co-author of the study, highlights how the research can be translated into real-world applications, potentially revolutionizing approaches to disease treatment. The proposed Griffiths-like cellular phase may not only deepen our understanding of these diseases but could also offer novel therapeutic strategies.
Recent findings tie phase separation to critical health issues such as cataracts and even the COVID-19 virus, emphasizing that the study of protein dynamics through the Griffiths-like phase lens may yield new paths for intervention. For example, the interaction between coacervation and viral proteins illustrates the delicate balance between beneficial and detrimental effects of protein droplet formation.
In concluding this exploration of protein compartmentalization and Griffiths phases, the research underscores the vital need for interdisciplinary collaboration in science. The work spearheaded by Souza and his colleagues is a testament to how blending fields such as physics, biology, and chemistry can lead to groundbreaking insights.
Through their innovative analytical approaches, they not only enrich our understanding of cellular dynamics but also set the stage for future research that could significantly impact health and disease management. This intersection of disciplines offers a promising glimpse into how fundamental concepts can be shared across the boundaries of scientific inquiry, steering the path towards new discoveries in both biology and medicine.

Leave a Reply