In both natural ecosystems and human-engineered technologies, the conversion of light into energy plays a pivotal role. Photosynthesis fuels the growth of plants and certain bacteria, while solar panels harness sunlight to generate electricity. At the heart of these processes lies electronic motion, a phenomenon that encompasses the transfer of charge at the molecular scale. Understanding the ultrafast dynamics of electron and charge transfer presents a formidable challenge to scientists, particularly when one considers the intricacies of quantum mechanics and molecular behavior. Recent advancements in measurement techniques have begun to shed light on this complex interplay, revealing fundamental insights that may lead to enhanced control of chemical processes in future applications.
The phenomenon of charge transfer after a molecule absorbs light occurs on an incredibly short timescale, ranging from femtoseconds (10^-15 seconds) to even attoseconds (10^-18 seconds). The ability to measure these rapid processes with extreme temporal resolution has become possible due to the emergence of innovative experimental techniques, including attosecond extreme-ultraviolet pulses generated by high-order harmonic sources and free electron lasers. Research efforts are focusing on understanding the initial stages of electron dynamics that follow photoionization events. Despite significant progress, capturing the minute details of charge migration remains a tantalizing challenge for contemporary scientists.
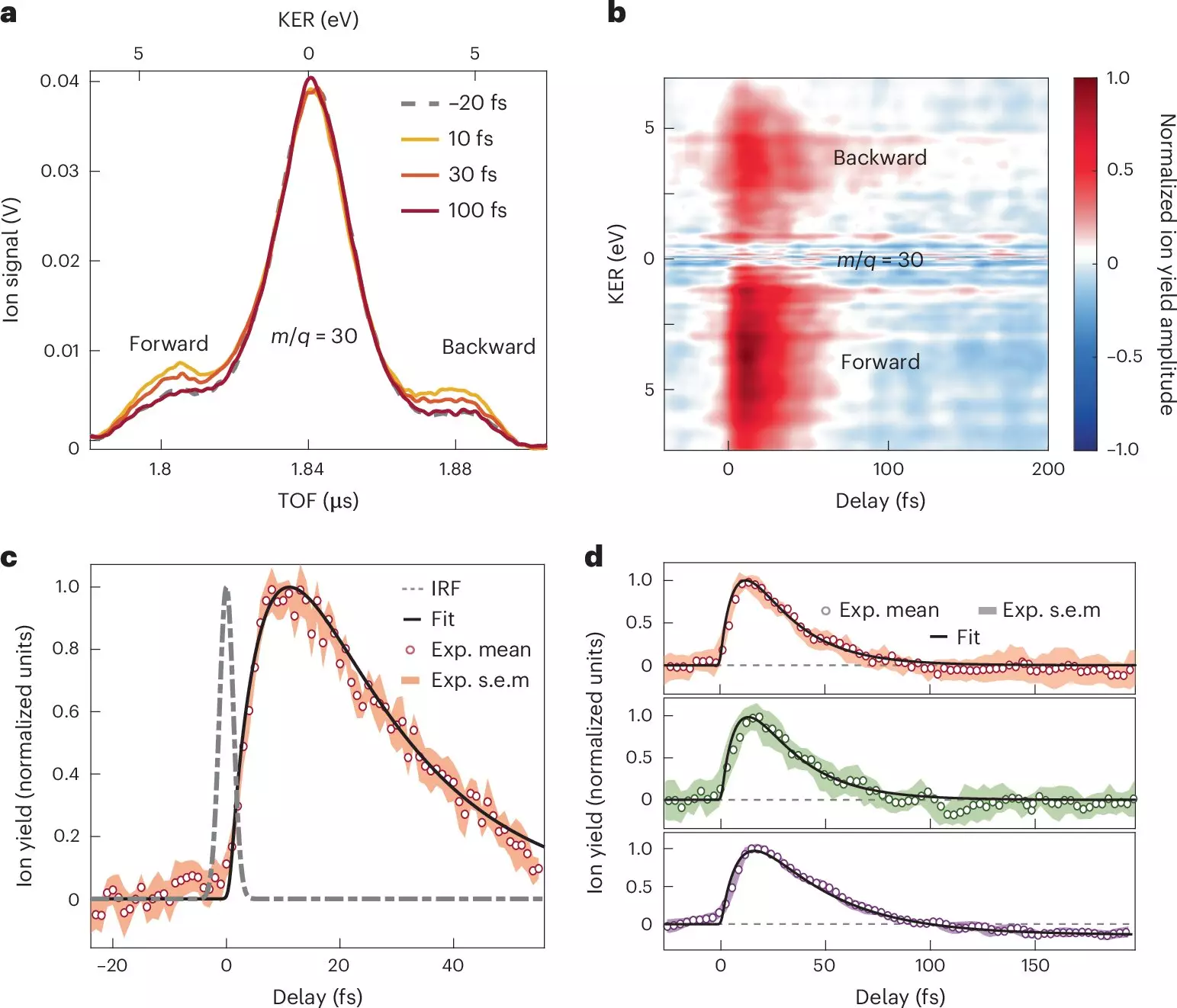
A compelling study published in *Nature Chemistry* by a collaborative research team from the Politecnico di Milano and several Madrid-based institutes marks a significant advancement in our understanding of ultrafast charge transfer. By employing attosecond extreme-ultraviolet pulses, the researchers have illuminated the intricate dynamics that govern the interactions between electrons and nuclei in donor-acceptor molecular systems. This work not only improves our comprehension of these essential processes but also opens avenues for engineering molecular properties to facilitate better performance in various applications, from organic photovoltaics to molecular electronics.
The researchers specifically focused on nitroaniline molecules and utilized a sophisticated combination of attosecond pulse techniques and advanced quantum chemistry models to observe the initial moments of charge transfer. Their findings revealed that the electron transfer from the amino group, acting as the electron donor, occurs in under 10 femtoseconds. This swift process is immediately followed by a relaxation of the molecular structure on the sub-30-femtosecond scale, demonstrating how the synchronized motion of the nuclei and electrons underpins the charge transfer phenomenon. These observations provide critical insights into how the electron-nuclear coupling influences the behavior of donor-acceptor molecular systems under light exposure.
The implications of these findings reach beyond mere scientific curiosity; they provide a substantial enhancement to our theoretical models that seek to predict charge migration in organic molecules. By detailing the timescales required for charge transfer and the corresponding structural adjustments within the molecule, this study enriches the understanding of chemical dynamics at a fundamental level. These insights possess the potential to inform the design of more efficient organic materials by guiding how we manipulate molecular structures to optimize electron transfer processes.
The investigation into ultrafast charge dynamics as presented by the researchers in this remarkable study is a prime example of how cutting-edge scientific tools can unveil the hidden intricacies of nature. By capturing the fleeting moments of charge transfer in nitroaniline, the study not only advances the frontiers of theoretical knowledge but also sets the stage for future exploration and innovation within the burgeoning field of attosecond science. As we continue to push the boundaries of our understanding, the dream of harnessing the principles of ultrafast charge transfer for practical applications in technology remains an exciting possibility. This research is a testament to the importance of exploring nature’s quantum processes, paving the way for breakthroughs that may reshape our technological landscape.

Leave a Reply