Recent advancements at TU Wien (Vienna) have ushered in a remarkable capability to generate laser-synchronized ion pulses with durations remarkably shorter than 500 picoseconds. This achievement, reported in *Physical Review Research*, is not merely an incremental step in physics but represents a significant overhaul in the methodologies we use to probe chemical processes occurring on material surfaces. By using this innovative technology, researchers can now observe and analyze dynamic chemical reactions while they are ongoing, providing insights previously limited to theoretical speculation.
The Challenges of Observing Rapid Chemical Reactions
Understanding chemical processes at the atomic level necessitates an equally rapid observational technique. In the same way that an extraordinary camera is essential for capturing fast-moving objects, physicists have long relied on extremely short laser pulses to visualize atomic movements. Traditional approaches, however, have often limited the scope of observation, as they primarily focused on the outcomes of reactions rather than the transient phenomena during those reactions. Prof. Richard Wilhelm from the Institute of Applied Physics at TU Wien emphasizes a fundamental challenge: while ion beams have been used for surface analysis and material modification for years, researchers typically only witness the aftermath of these interactions rather than their real-time evolution.
At the heart of the challenge lies the duration of ion pulses. Until the advent of this new method, ion pulses lacked the brevity needed to capture rapid changes at the surface level. The newly generated ion pulses, each lasting less than 500 picoseconds, start to bridge that significant temporal gap. Though this still falls short of the enormity of time resolution provided by attosecond pulses, it enters a regime ideally suited for surface studies.
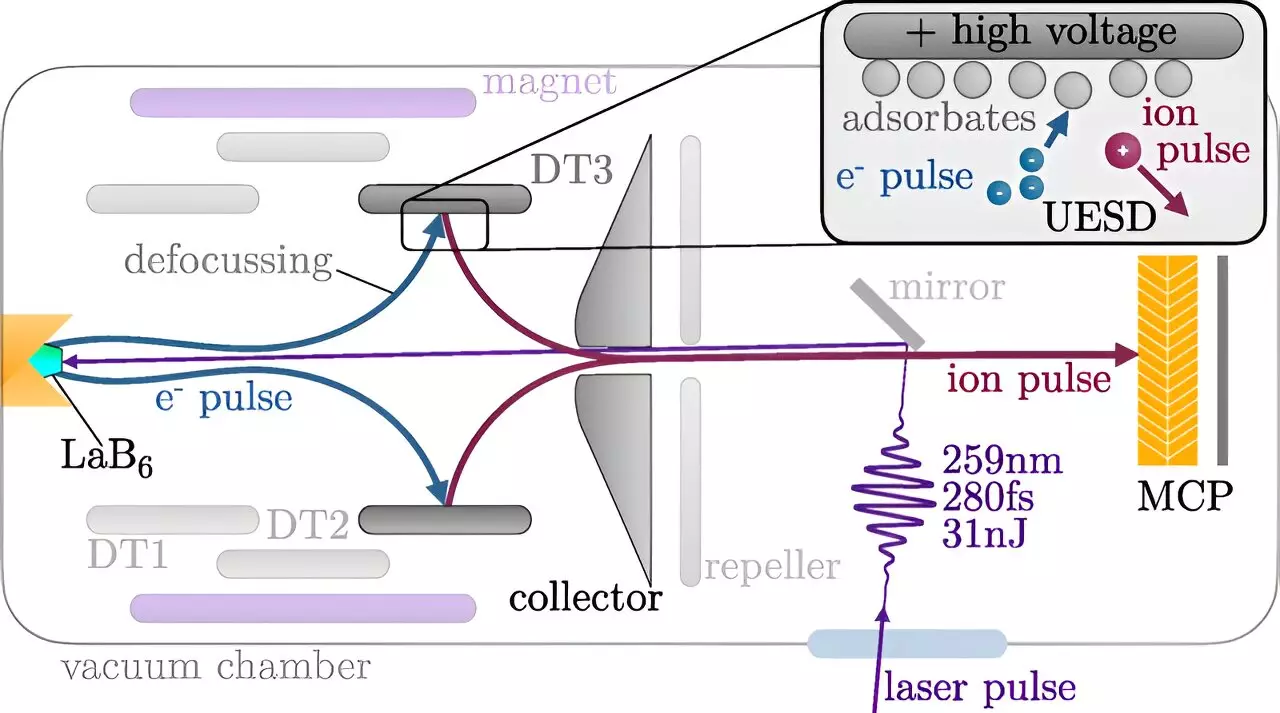
So, how does TU Wien’s new methodology succeed where previous efforts faltered? The generation of these ultrashort ion pulses utilizes a complex multi-stage process involving laser and electron interactions. Initially, a laser pulse is directed at a cathode, where it triggers the release of electrons. These energized electrons then collide with a stainless steel target, expelling atoms from its surface — specifically, hydrogen and oxygen atoms.
This step is crucial, as it initiates the creation of charged particles. As these atoms disperse, some remain neutral while others become ionized. Researchers can employ electric fields to differentiate and select which ions will be utilized for further analysis, thereby directing them with precision onto the target surface.
Moreover, this novel method’s temporal control allows scientists to orchestrate the timing of the ion impulses, effectively probing the reaction dynamics while they take place — akin to capturing a fast-action photograph where the moments of transition are as significant as the final scene. Wilhelm’s assertion that this capability opens up multiple avenues for investigation — such as studying surface interactions concurrent with laser-activated reactions — highlights the potential breadth of this innovation.
Presently, the analysis is centered around the simplest ion variations, such as protons. Nevertheless, the versatility of the technique permits the generation of other types of ions including carbon or oxygen, fundamentally broadening the scope of research applications. With precise control over which atoms adhere to the stainless steel surface, researchers can tailor their experimental setup to yield a variety of ions for analysis. They may even venture into generating neutral or negatively charged ion pulses, thereby expanding the toolkit available for surface chemistry investigations.
Excitingly, there are already plans to refine this technology even further. By manipulating electromagnetic fields, researchers aim to increase the efficiency and intensity of the ion pulses, with a vision of shortening the duration of these pulses even more. Wilhelm optimistically states that this advances the frontier of ultrafast investigations, offering new insights into phenomena that were previously elusive.
The convergence of this method with established ultrafast electron microscopy technology propels the potential of this research to new heights. By synergizing these approaches, researchers can unlock deeper understandings of the complex interplay between physics and chemistry at material surfaces, paving the way for innovations in various fields — from catalysis to materials science, and beyond.
As we stand on the brink of this new era of research, the implications of laser-synchronized ion pulses could redefine our understanding of chemical processes, revealing the intricacies of atomic interactions as they happen. Through meticulous exploration and continued advancements, this groundbreaking work from TU Wien signals a transformative period for experimental physics and chemistry, one that promises to rewrite what we thought was possible in surface analysis.

Leave a Reply